14 Mar EU AI Act 2024/1689 and its impact on Medical Devices

Understanding the new EU AI Act 2024/1689:
Legislative Background:
The legislation background of this AI act includes the Treaty on the Functioning of the European Union (TFEU) and specific regulations such as Regulation (EU) 2024/1689 of the European Parliament and of the Council. It aims to provide a comprehensive framework for the regulation of AI systems within the European Union
Legislative Publication:
The European AI Act 2024/1689 was officially published on July 12, 2024, in the Official Journal of the European Union. With this publication, the law becomes mandatory.
Purpose of the Regulation:
The regulation aims to improve the functioning of the internal market by establishing a uniform legal framework for the development, marketing, and use of artificial intelligence (AI) systems in the EU. It emphasizes promoting human-centric and trustworthy AI while ensuring a high level of protection for health, safety, and fundamental rights.
Amendments to Existing Legislation:
The act includes amendments to Directive (EU) 2020/1828 and other Union legislative acts, demonstrating its wide-reaching impact on existing legal frameworks.
Different AI types:
there are different types of AI systems categorized as high-risk AI systems. These include AI systems used in areas such as biometrics (remote biometric identification systems, biometric categorization, emotion recognition), critical infrastructure (safety components in critical digital infrastructure, road traffic, water, gas, heating, or electricity supply), education and vocational training (access determination, learning outcome evaluation, education level assessment, behavior monitoring during tests), employment (recruitment, job analysis, candidate evaluation, work-related decisions, performance monitoring), and access to essential private and public services (evaluation of eligibility).
High-Risk AI Systems:
The document mentions the importance of addressing the risks associated with high-risk AI systems, ensuring they meet mandatory requirements to maintain trustworthiness and protect users.
The AI Act introduces a comprehensive framework to regulate high-risk AI systems, ensuring the safety and protection of individuals’ health and fundamental rights. For medical device companies, understanding the risk levels outlined in the AI Act is crucial to compliance and successful market entry.
The Act emphasizes the identification, analysis, and mitigation of risks associated with high-risk AI systems used in healthcare settings. It requires companies to assess known and foreseeable risks, estimate potential emergent risks, and implement targeted risk management measures to address these concerns effectively.
Furthermore, the Act highlights the importance of transparency and provision of information to deployers, ensuring that AI systems are designed in a way that enables users to interpret outputs and use them appropriately. This is particularly relevant for medical device companies, where clear and concise instructions for use are essential for safe and effective deployment of AI systems.
Testing of high-risk AI systems in real-world conditions is also a key aspect of the Act, allowing providers to identify appropriate risk management measures and ensure compliance with regulatory requirements. This testing must be conducted to assess the consistency of AI systems for their intended purpose and their alignment with regulatory standards.
Compliance Timeline:
AI systems already on the market or in service before the regulation’s entry into force must comply with the new requirements by 31 December 2030, indicating a transition period for existing AI systems to adapt to the new regulations.
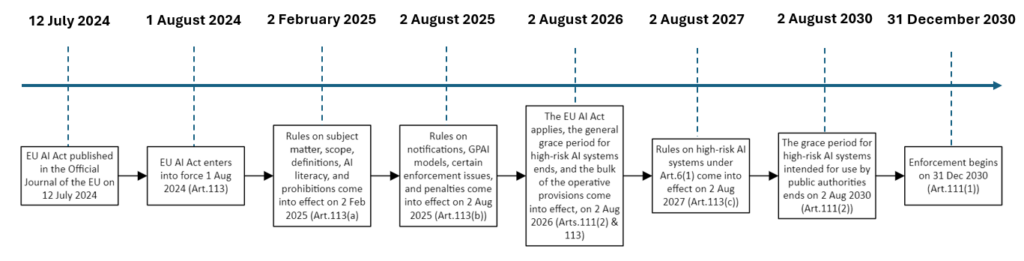
Detailed timelines:
- AI systems which are components of large-scale IT systems placed on the market or put into service before 2 August 2027 should be brought into compliance with the Regulation by 31 December 2030.
- Providers of high-risk AI systems are encouraged to start complying voluntarily with the relevant obligations of the Regulation during the transitional period.
- The Regulation should apply from 2 August 2026, with prohibitions and general provisions coming into effect from 2 February 2025 to address unacceptable risks associated with AI use in certain ways.
- Infrastructure related to governance and the conformity assessment system should be operational before 2 August 2026, with provisions on notified bodies and governance structure applying from 2 August 2025.
- Obligations for providers of general-purpose AI models should apply from 2 August 2025, and codes of practice should be ready by 2 May 2025 to enable providers to demonstrate compliance on time.
This act represents a significant step in regulating AI technologies within the EU, focusing on safety, transparency, and ethical considerations to foster innovation while protecting citizens’ rights and well-being.
Positive Impacts of the EU AI Act on Medical Devices:
1. Enhanced Patient Safety: By setting stringent standards for AI in medical devices, the act can significantly improve patient safety. Ensuring that AI systems are reliable and safe before they are deployed in healthcare settings can prevent potential harm and improve clinical outcomes.
2. Increased Trust in AI Technologies: Regulatory oversight can increase trust among healthcare professionals and patients in AI technologies. Knowing that AI-based medical devices have undergone rigorous evaluation can boost their adoption and integration into healthcare practices.
3. Stimulating High-Quality Innovations: The act can encourage developers to focus on high-quality, impactful innovations that meet the regulatory standards. This can lead to the development of more advanced, effective, and safe medical devices, ultimately benefiting patients and the healthcare system.
4. Harmonization Across the EU: A unified regulatory framework can facilitate the smoother introduction of AI medical devices across the EU market. This harmonization can reduce barriers to entry for innovators and ensure that patients across the EU have access to the latest technologies.
Potential Limitations of the EU AI Act on Medical Devices:
1. Potential for Stifling Innovation: Regulatory requirements can be seen as burdensome, especially for startups and smaller companies with limited resources. The complexity and cost of compliance may hinder the pace of innovation and discourage some developers from pursuing potentially groundbreaking ideas.
2. Risk of Overregulation: There’s a delicate balance between ensuring safety and stifling innovation. Overly stringent regulations could slow down the development and deployment of AI technologies in medical devices, potentially delaying the benefits they can offer to healthcare.
3. Adaptability to Rapid Technological Advances: The fast-paced nature of AI development poses a challenge to regulatory frameworks, which may struggle to keep up. There’s a risk that the regulations may become outdated quickly, limiting their effectiveness and relevance.
4. Variability in Implementation: While the act aims for harmonization, the interpretation and implementation of the regulations could vary across different EU member states. This variability might create inconsistencies and uncertainties for developers looking to enter multiple EU markets.
Conclusion:
The EU AI Act has the potential to significantly impact the field of medical devices, with both positive implications for patient safety and trust and potential challenges related to innovation and regulatory compliance. A well-balanced approach, which includes ongoing dialogue between regulators, developers, healthcare professionals, and patients, is essential to maximize the benefits of AI in healthcare while minimizing the risks. Creative solutions, such as regulatory sandboxes or innovation hubs, could also be explored to support the development of safe and effective AI technologies in a manner that fosters innovation.
Regulatory Intelligence Service:
We are helping companies with being up to date with medical device regulation globally. Learn more and join our exclusive service by clicking HERE.




No Comments