Quick Link: Country Overview | Why MDSAP | Key Topics of MDSAP
MDSAP
Medical Device Single Audit Program (MDSAP)
MDSAP stands for Medical Device Single Audit Program. It’s a program that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system that satisfies the requirements of multiple regulatory jurisdictions. Audits are conducted by Auditing Organizations (AO), which are authorized by the participating Regulatory Authorities (RAs) to conduct MDSAP audits.
The MDSAP is an audit program that is composed of the following five jurisdictions: USA, Australia, Canada, Japan and Brazil.
Country Overview:
USA – FDA: Food and Drugs Administration
The quality management requirements are based on QSR – 21 CFR Part 820.
Australia – TGA: Therapeutic Goods Administration
The quality management requirements are based on TGA regulation (TG(MD)R Sch3).
Brazil – ANVISA: Agência Nacional de Vigilância Sanitária
The quality management requirements are based on RDC ANVISA 16/2013.
Canada – Health Canada:
The quality management requirements are based on ISO 13485:2016.
Japan – MHLW & PMDA: Ministry of Health Labor and Welfare & Pharmaceuticals and Medical Devices Agency
The quality management requirements are based on MHLW Ordinance No. 169.
MDSAP Offical Observers (Not part of the MDSAP Program):
- Europen Union (EU) – (The quality management requirements are based on ISO 13485:2016)
- United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA)
- The World Health Organization (WHO) Prequalification of In Vitro Diagnostics (IVDs) Programm
MDSAP Affiliate Members (Not part of the MDSAP Program):
- Argentina’s National Administration of Drugs, Foods and Medical Devices (ANMAT)
- Ministry of Health of Israel (GOV)
- Kenya’s Pharmacy and Poisons Board (ORG)
- Republic of Korea’s Ministry of Food and Drug Safety (MFDS)
- Federal Commission for Protection from Sanitary Risks (COFEPRIS) of Mexico
- Singapore’s Health Sciences Authority (HSA)
- Taiwan Food and Drug Administration (TFDA)
What is MDSAP?
The Medical Device Single Audit Program (MDSAP) represents an innovative, global approach to regulatory oversight of medical devices. It allows for a single audit, conducted by an authorized Auditing Organization (AO), to assess a medical device manufacturer’s quality management system against the requirements of multiple regulatory jurisdictions.
Under the MDSAP framework, a rigorous, comprehensive inspection is performed, covering all aspects of the medical device’s lifecycle, from design and development, to production, post-production, and distribution. It ensures a uniform interpretation of regulations and standards, thereby promoting a consistent level of quality and safety worldwide.
Participating Regulatory Authorities (RAs), including those from Australia, Brazil, Canada, Japan, and the United States, mutually recognize the outcomes of these audits. This streamlines the regulatory process, reduces administrative burdens, and fosters international collaboration, while maintaining a high level of public health protection.
In summary, the MDSAP serves as an efficient, cost-effective model that enhances global regulatory alignment and ensures compliance with stringent quality and safety standards, thereby improving the reliability and efficacy of medical devices in the marketplace.
Why MDSAP:
The Medical Device Single Audit Program (MDSAP) offers a multitude of advantages for medical device manufacturers, which can contribute significantly to their efficiency, cost management, and overall success in the marketplace. Here are some of the key benefits:
- Streamlined Regulatory Compliance: MDSAP allows manufacturers to undergo a single audit that covers the requirements of multiple regulatory authorities. This reduces the need for multiple audits by different regulatory bodies, saving both time and resources.
- Market Access: By participating in the MDSAP, manufacturers can demonstrate compliance with the requirements of all participating countries. This can help to accelerate access to new markets and reduce barriers to international trade.
- Enhanced Productivity: By reducing the time and effort spent on preparing for and undergoing multiple audits, manufacturers can focus more on their core activities, such as research and development, product improvement, and customer service.
- Reduced Risks: The MDSAP process provides a comprehensive review of the manufacturer’s quality management system. This can help to identify potential issues early, reducing the risk of non-compliance and potential recall or market withdrawal situations.
- Improved Quality: The rigorous MDSAP audit promotes a high standard of quality management across the organization, which can lead to improved product quality, greater customer satisfaction, and a stronger competitive position.
- Transparency and Predictability: The MDSAP provides a clear and predictable regulatory path, making it easier for manufacturers to plan and manage their regulatory activities effectively.
The MDSAP is, therefore, an excellent tool for medical device manufacturers seeking to enhance their regulatory compliance, improve efficiency, and access new markets.
Benefits:
The main benefits of this program are:
- Reduction of external Audits
- Better market access
- Positive image of your Company and quality
Risks:
Risk of the MDSAP program could be:
- Transparency
- Follow-up Audits
- Audit not passed – Sales stop in all five countries
Key Topics of MDSAP:
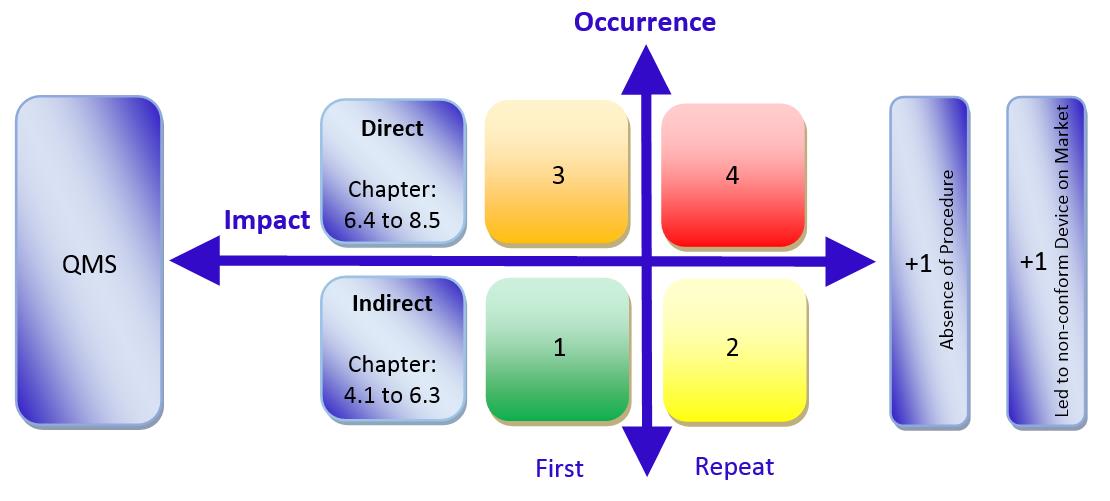
Audit Grading of nonconformities:
The MDSAP grading system has five grading levels and each nonconformity will be calculated with the following system:
Indirect nonconformities (Chapter 4.1 to 6.3) are the low-risk issues and normally starting with grading level 1 (first nonconformity) or grading level 2 (repeated nonconformity). Depends on the deviation, additional gradings can be put on top. For example, the deviation has an absence of procedure or it ends in a non-conformity product on the market (+1 Level each).
Direct nonconformities (Chapter 6.4 to 8.5) are the high-risk issues and starting at grading level 3 (first nonconformity) or grading level 4 (repeated nonconformity). Depends on the deviation, additional gradings can be put on top. For example, the deviation has an absence of procedure or it ends in a non-conformity product on the market (+1 Level each).
When you will not pass the MDSAP audit:
If the Auditing Organization finds three or more 4 levels or one or more 5 levels.
Timeline after the Audit:
The timeline below shows steps to be taken to improve processes and procedures following the audit.
How to implement the MDSAP requirements?
Implementing the Medical Device Single Audit Program (MDSAP) within your organization using an Excel checklist involves several steps. Before starting, ensure you understand the requirements of MDSAP, which includes compliance with ISO 13485:2016 (the international standard for medical device quality management systems), and any specific regulations in participating countries.
Here’s a simplified step-by-step guide:
- Understand MDSAP Requirements: Review the MDSAP Companion Document and Audit Model. These provide an overview of what auditors will inspect during an MDSAP audit.
- Use our MDSAP Excel Checklist: This checklist should include the relevant clauses from ISO 13485, as well as the requirements from the MDSAP Companion Document and Audit Model.
- Perform a Gap Analysis: Use your MDSAP Excel checklist to perform a gap analysis. Go through each requirement and evaluate if your company is currently meeting the requirement or not. Record your findings in the Compliance Status column and provide any relevant notes or evidence in the Evidence/Comments column.
- Create a Plan of Action: For any gaps identified, write down the action needed to address them, assign a responsible person, and set a deadline for the action.
- Implement the Changes: Follow your plan of action and monitor the progress. Update the checklist as changes are implemented.
- Prepare for MDSAP Audit: Once you have closed all the gaps and updated your checklist accordingly, prepare for the MDSAP audit. Make sure all necessary documents are available and staff are trained on the requirements.
- Perform Internal Audits: Regular internal audits will help ensure ongoing compliance and identify any new gaps that may arise.
- Continuous Improvement: MDSAP should be considered a continuous process, with regular review and updates to the Excel checklist.
Please note that these are just simplified steps. Actual implementation may require a deeper understanding of MDSAP requirements, a structured approach, professional expertise, and consistent efforts.
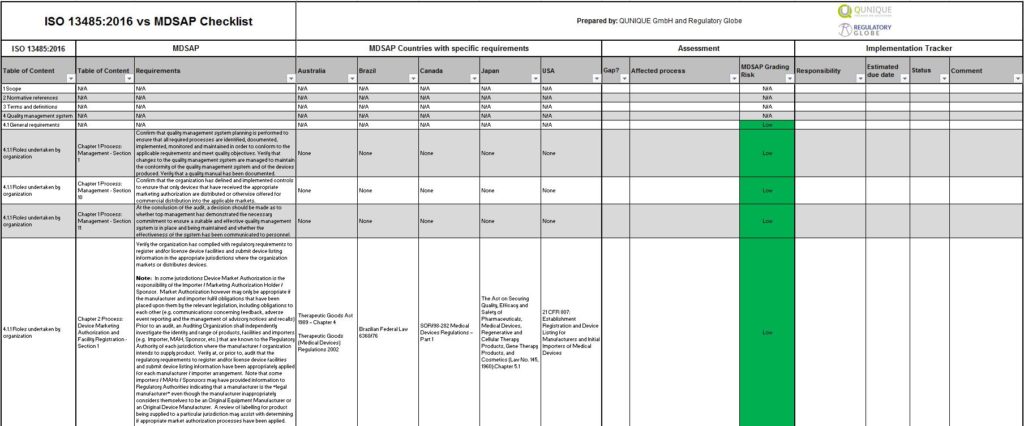
MDSAP Audit Checklist Excel
Our MDSAP integration solution:
MDSAP vs ISO 13485:2016 Checklist – Click here to get our test version.
Current valid Audit Approach document – MDSAP AU P0002.008 (01. April 2023)
Freshly updated MDSAP Audit Approach document – MDSAP-AU-P0002.008:
Changes from version 007 to 008:
Audit Sequence:
- Added the option to audit the Production and Service Controls process following the Measurement, Analysis and Improvement followed by the Design and Development process as a reasonable deviation from the MDSAP audit sequence on page 9
Management Process:
Task 10
- added clarification that AOs should also consider private-labelled medical devices when verifying that products that have received marketing authorization are imported or sold in Canada.
Measurement, Analysis, and Improvement Process:
Task 6
- added Canadian regulatory reference.
Medical Device Adverse Events and Advisory Notices Reporting
Task 1
- removed hyperlinks to Canadian guidance documents.
Task 2
- Correct hyperlink to webpage for TGA Recalls
Design and Development Process:
Task 10
- Removed the Australian specific requirement. Standards that are used to demonstrate compliance with the Australian Essential Principles are not mandatory. Production and Service Controls
Task 24
- Removed the phrase “as per ISTA 2A”
Annex 1
- Removed a reference to an Essential Principles Checklist (See Annex 1 – Additional country-specific requirements, Australia – TGA, Auditing Technical Documentation, for a description of the use of an Essential Principles Checklist)