03 Aug SaMD
SaMD – Software as a Medical Device
This article provide a-depth overview of Software as a Medical Device (SaMD) and its regulatory landscape, focusing on FDA and EU regulations.
Understanding SaMD
Software as a Medical Device (SaMD) is defined by the International Medical Device Regulators Forum (IMDRF) as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device”. This definition is crucial as it distinguishes SaMD from software integrated into hardware medical devices (Software in a Medical Device or SiMD).
Key characteristics of SaMD include:
- Independence from hardware medical devices
- Intended for medical purposes (e.g., diagnosis, treatment, monitoring)
- Ability to run on general-purpose computing platforms
Regulatory Framework in the US (FDA)
The FDA regulates SaMD as medical devices, classifying them based on their intended use and risk level.
Classification:
- Class I: Low risk, subject to general controls.
- Class II: Moderate risk, requiring general controls and special controls, often cleared through the 510(k) premarket notification process.
- Class III: High risk, subject to general controls, special controls, and premarket approval (PMA), which involves rigorous review of clinical data.
The classification determines the regulatory pathway and requirements for market approval. Most SaMD fall under Class II, requiring a 510(k) premarket notification.
Key FDA Guidance Documents:
- Policy for Device Software Functions and Mobile Medical Applications: This guidance outlines the FDA’s regulatory oversight of software functions that meet the definition of a medical device and could pose risks to patient safety if malfunctioning.
- Clinical Decision Support Software: Provides guidelines for software that assists healthcare providers in making clinical decisions.
- Software as a Medical Device defined by the International Medical Device Regulators Forum (IMDRF)
Artificial Intelligence and Machine Learning:
The FDA is actively working on a regulatory framework for AI/ML-based SaMD. They released the “Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan“.
Regulatory Framework in the EU
In the EU, SaMD is regulated under the Medical Device Regulation (MDR 2017/745) and In Vitro Diagnostic Regulation (IVDR 2017/746).
Classification:
The EU uses a risk-based classification system with four classes:
- Class I
- Class IIa
- Class IIb
- Class III
Most SaMD fall under Class IIa or higher, requiring involvement of a Notified Body for conformity assessment.
Key Regulatory Requirements:
- EU MDR 2017/745 or EU IVDR 2017/746
- Quality Management System (ISO 13485)
MDCG Guidance:
The Medical Device Coordination Group (MDCG) has issued several guidance documents relevant to SaMD, including:
- MDCG 2019-11 on Qualification and Classification of Software
- MDCG 2019-16 Rev, 1 – Guidance on cybersecurity for medical devices
- MDCG 2020-1 – Guidance on clinical evaluation (MDR) / Performance evaluation (IVDR) of medical device software
- MDCG 2023-4 – Medical Device Software (MDSW) – Hardware combinations Guidance on MDSW intended to work in combination with hardware or hardware components
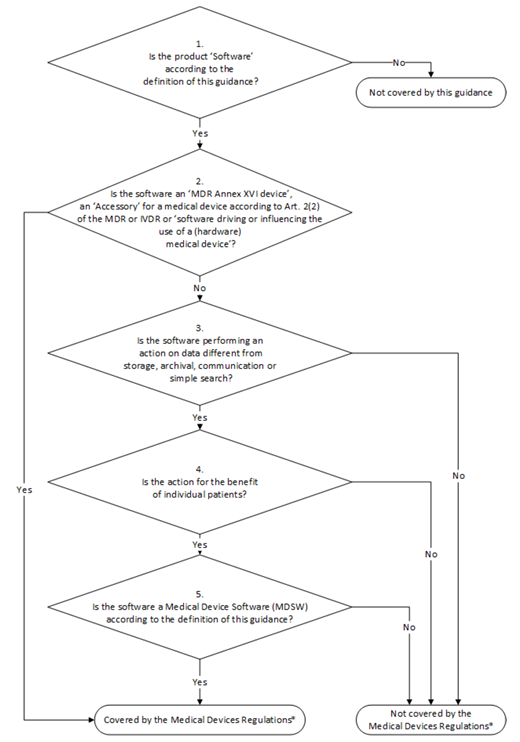
SaMD Decision Tree according to EU MDR and EU IVDR
:
Emerging Trends and Challenges
- AI/ML Integration: As AI and ML become more prevalent in SaMD, regulators are adapting their approaches. Stay informed about evolving guidelines for AI/ML-based SaMD.
- Real-World Evidence: Both FDA and EU regulators are increasingly accepting real-world evidence for regulatory decisions. Consider how to leverage this in your clinical evaluation strategy.
- Interoperability: As healthcare systems become more interconnected, ensure your SaMD can safely and effectively integrate with other systems.
- Rapid Iteration: Balance the need for quick software updates with regulatory requirements for change management and validation.
Conclusion:
In conclusion, navigating the regulatory landscape for SaMD requires a thorough understanding of both software development and medical device regulations. Stay informed about regulatory updates, engage with regulators early in the development process, and maintain a strong focus on patient safety and product quality throughout the SaMD lifecycle.
Regulatory Intelligence Service:
We are helping companies with being up to date with medical device regulation globally. Learn more and join our exclusive service by clicking HERE.




No Comments