QMSR Gap-Assessment Tool
QSR → QMSR & ISO 13485 – fast, clear, traceable
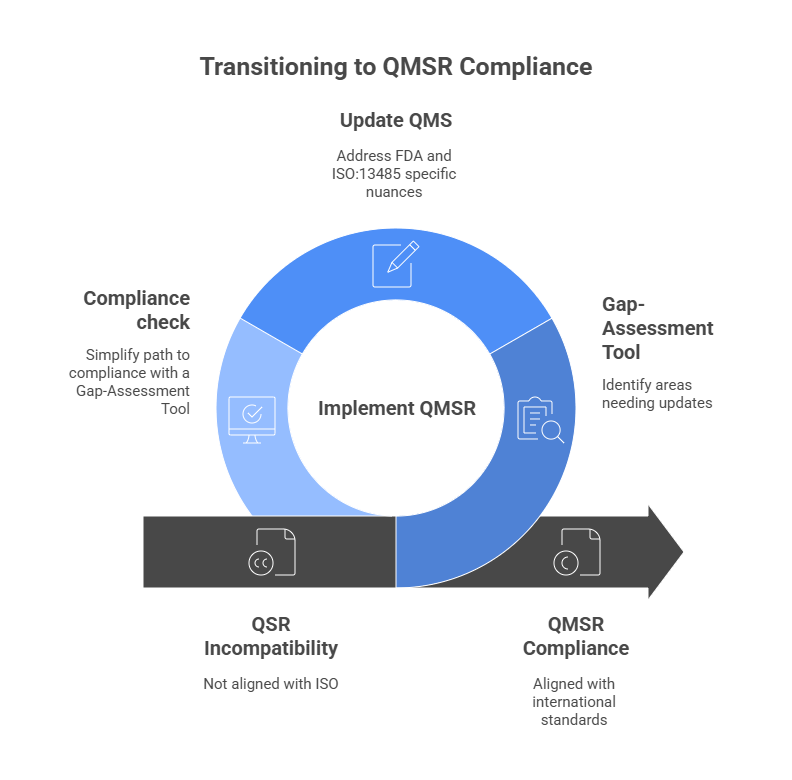
From February 2, 2026, the QMSR will be in force in the United States. Our online tool shows you clearly and systematically what is changing and includes a complete cross-reference mapping across all requirements.
How it works
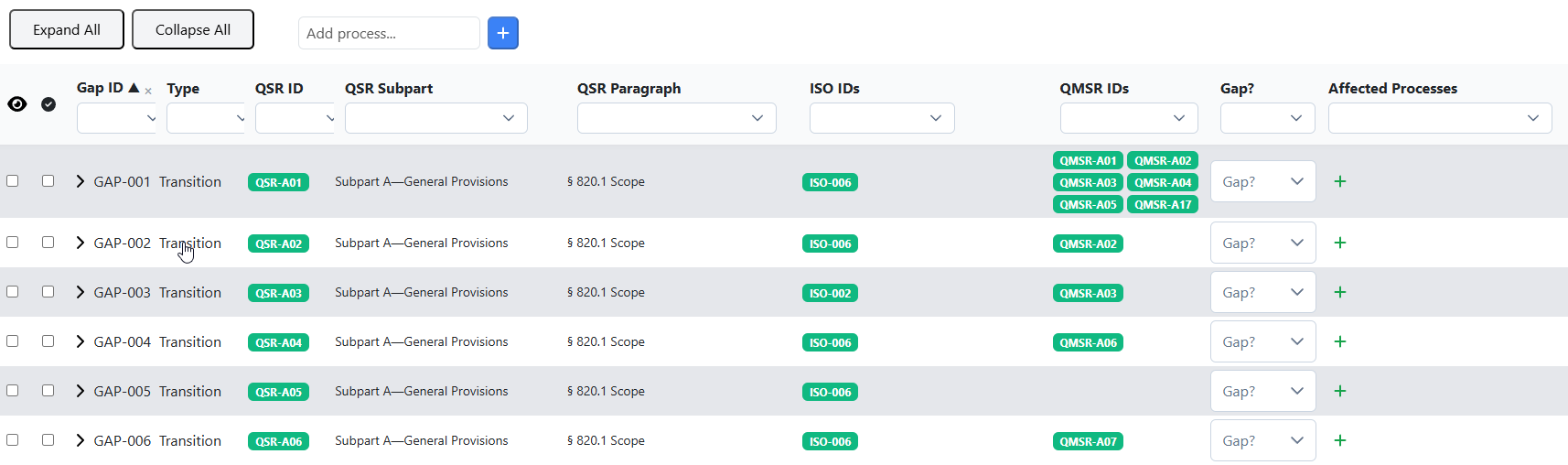
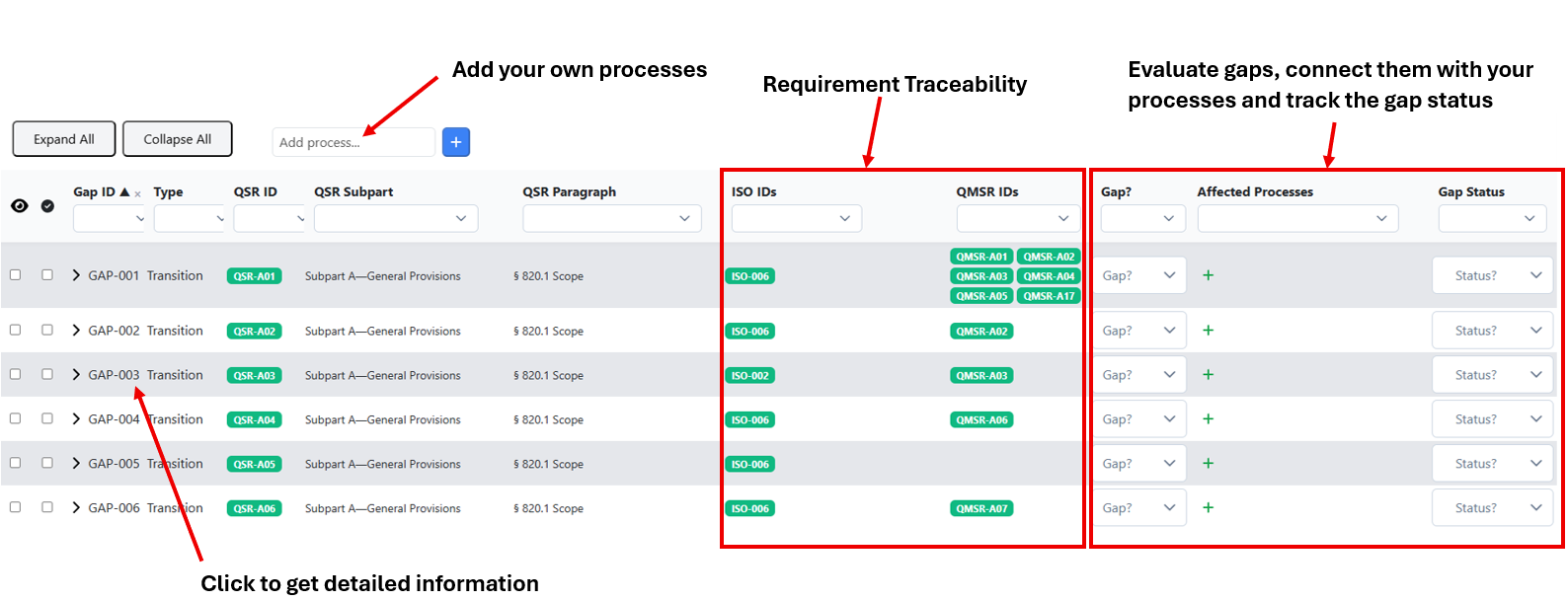
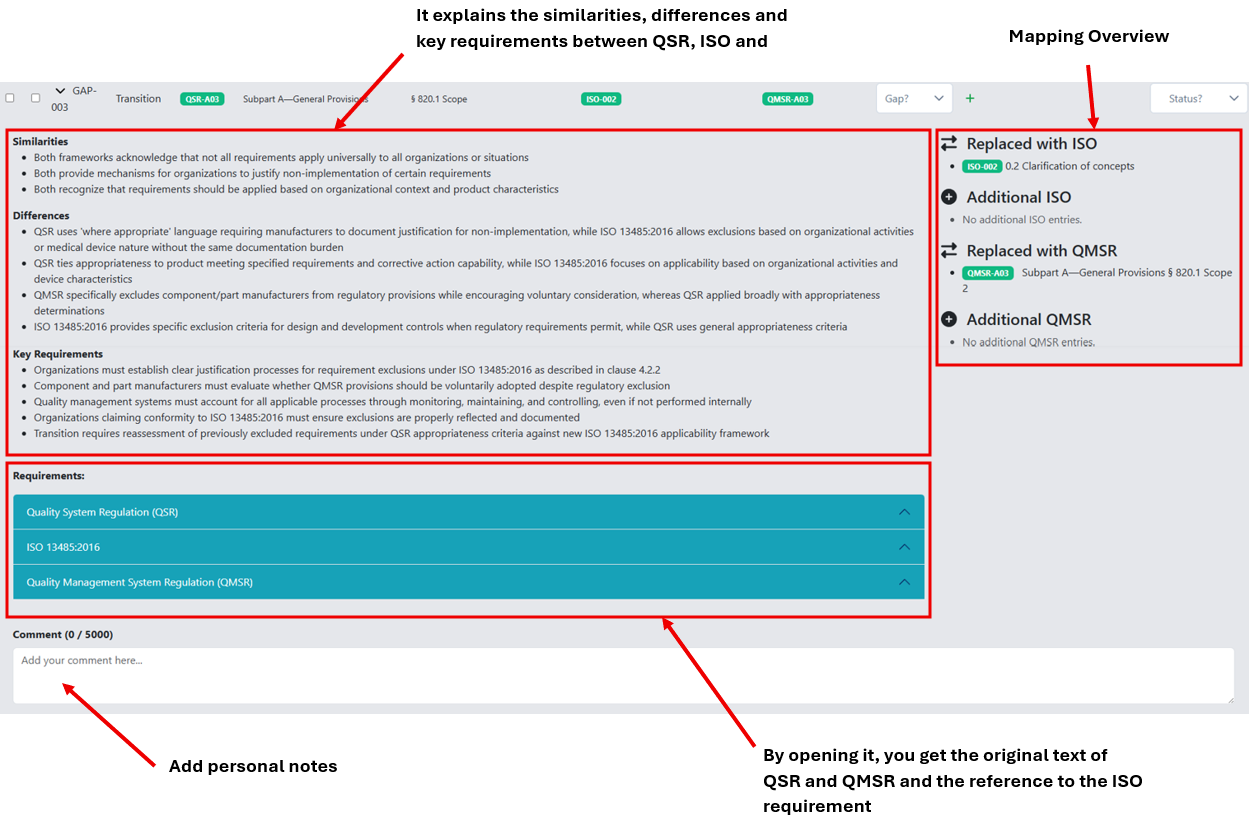
In the following image descriptions, you will see at a glance where to find things and what you can do.
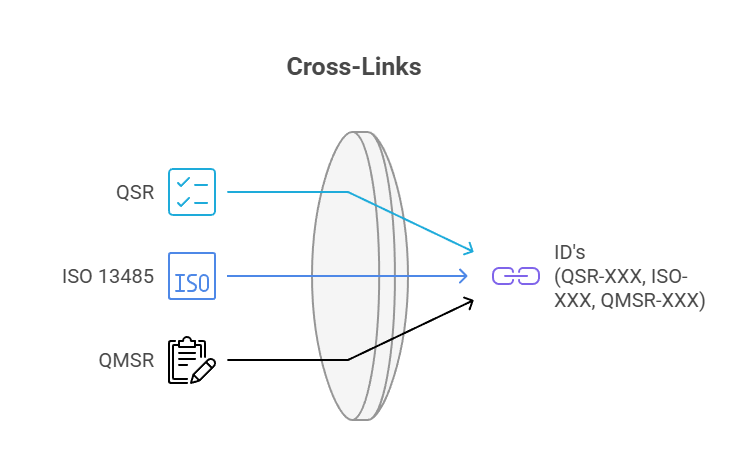
All requirements are cross-referenced via IDs (QSR-XXX, ISO-XXX, QMSR-XXX), so you can instantly see where the regulations and the ISO standard have similar requirements.
When you open a requirement, you will get additional, valuable information to support your gap assessment, especially detailed notes on similarities, differences, and key requirements.
The video below explains how the tool works, step by step.
FAQ
We’re already ISO 13485 compliant — do we still need this tool?
Yes. QMSR incorporates ISO 13485:2016 by reference, but includes U.S.-specific requirements as well as terminology/process changes. The tool shows exactly where action is needed.
Why not just build our own Excel list?
You get a ready-made, curated mapping with labels, short comments, and cross-links — usable immediately, without weeks of internal effort.
What does the trial include?
The trial displays 10 rows as examples. The full version contains 134 rows.
Is there support/onboarding?
Yes. 15-min demo: Book a call
Email support: info@regulatoryglobe.com
How to buy the full version?
How to buy the full version?
Create a free account – after you have logged in, you will see the green buy button on the top right
Which payment methods are available?
Credit card
Next Step
Shorten your path to QMSR conformity.
(Contact: info@regulatoryglobe.com • We typically reply within 1 business day (excluding weekends and public holidays))