QSR to QMSR Gap-Assessment Tool
499.00 CHF
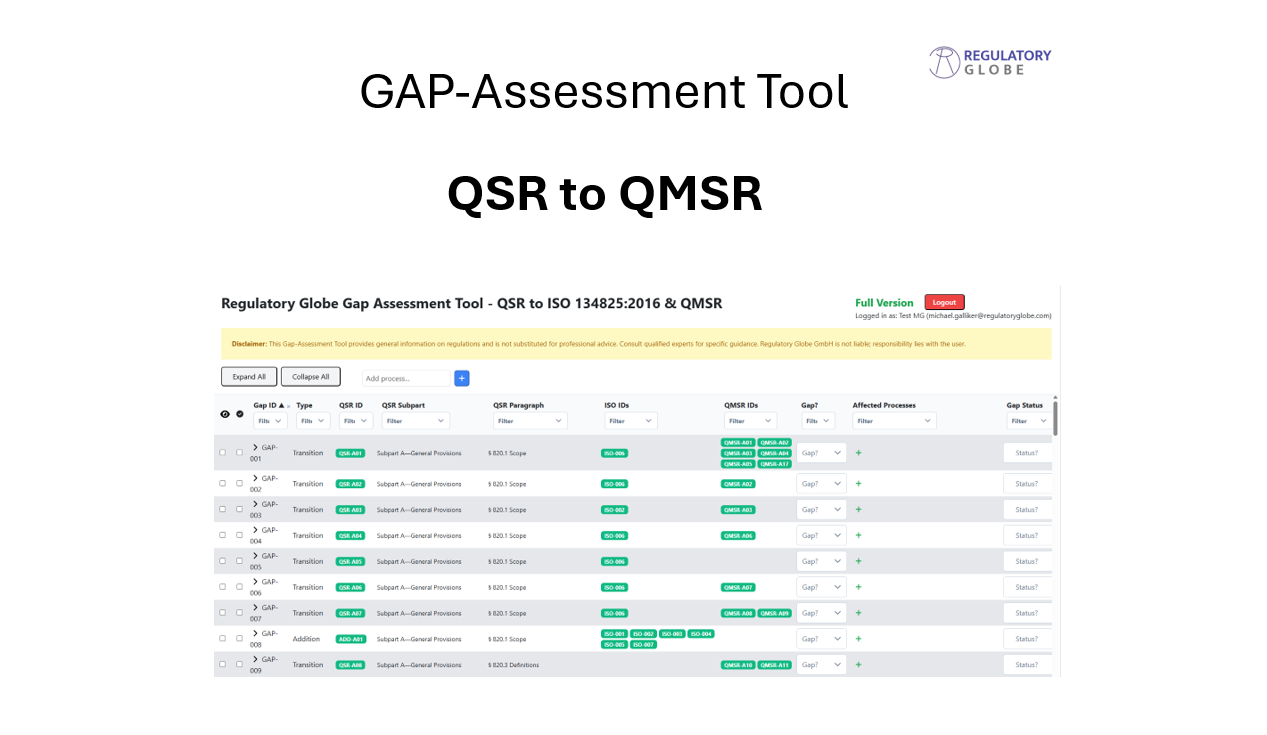
This QSR to QMSR Gap Assessment Tool is a cloud based tool designed to make your transition smooth, fast, and audit-ready. Developed by regulatory experts, this tool identifies all gaps between your current QSR-based system and the new QMSR—including ISO 13485 requirements and FDA-specific additions.
How it works:
Test Version
Benefits:
- Avoid costly noncompliance
- Save time with a ready-to-use compliance map
- Easy to sort by specific requirements and gaps
What you get:
- A clear, section-by-section gap analysis
- Mapping from QSR to ISO 13485 and QMSR
- Coverage of FDA-specific requirements not in ISO 13485
Our Payment Partners:

Description
Document Format:
- Cloude based Tool
Language:
- English
Reference Documents:
- QSR
- QMSR
- References to ISO 13485:2016
Reviews (0)
Only logged in customers who have purchased this product may leave a review.



Reviews
There are no reviews yet.