04 Aug From QSR to QMSR
From QSR to QMSR: What’s Changing and How to Prepare
The FDA is officially replacing its long-standing Quality System Regulation (QSR, 21 CFR Part 820) with the new Quality Management System Regulation (QMSR) — and the clock is ticking.2017/745, MDCG 2019-11, MDCG 2020-16, and MDCG 2021-24:
What’s Changing?:
The QMSR, effective February 2, 2026, brings the U.S. in line with international standards by incorporating ISO 13485:2016 by reference. This means that manufacturers who already comply with ISO 13485 will find many of the requirements familiar — but not identical.
Here’s what you need to know:
Alignment, not duplication – The QMSR aims to eliminate redundant efforts for manufacturers marketing both in the U.S. and internationally.
ISO 13485 becomes the foundation – But the FDA adds clarifications to ensure alignment with the FD&C Act
Some QSR terms (like DMR, DHR, DHF) are no longer used – But their elements still need to be documented under ISO 13485 clauses.
Compliance is still mandatory – Even if you’re ISO 13485 certified, you’ll need to address FDA-specific nuances.
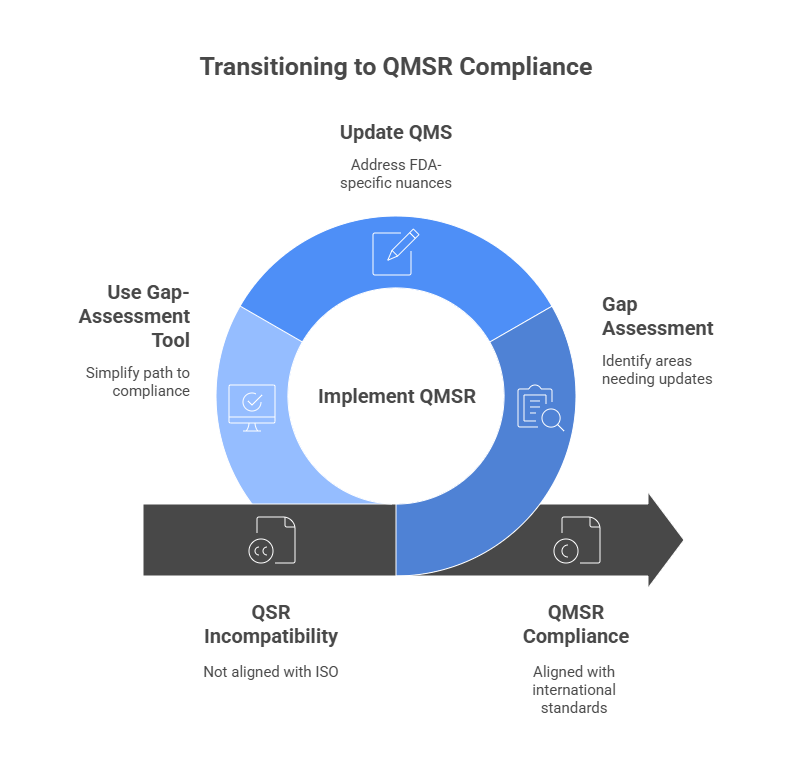
How Can You Prepare?
Start with a gap assessment between your current QMS (QSR or ISO 13485-based) and the QMSR. That’s where our Gap-Assessment Tool comes in.
With just a few clicks, our tool:
- Maps your current QMS against QMSR requirements
- Highlights areas needing updates or clarification
- Provides actionable steps to get you compliant by 2026
Whether you’re QSR-based, ISO 13485 certified, or somewhere in between — our tool simplifies your path to compliance.
Ready to get started? Test Tool here:




No Comments